The virtual sensors allow data fusion from various vehicle sensors and provide a prediction for measurement that is hard or too expensive to measure in another way or in the case of demand on continuous detection. The actual mass of 12 C is 12 daltons with one dalton is equal to 1661 10-24 g.

Relative Molecular Mass Inside Chemistry
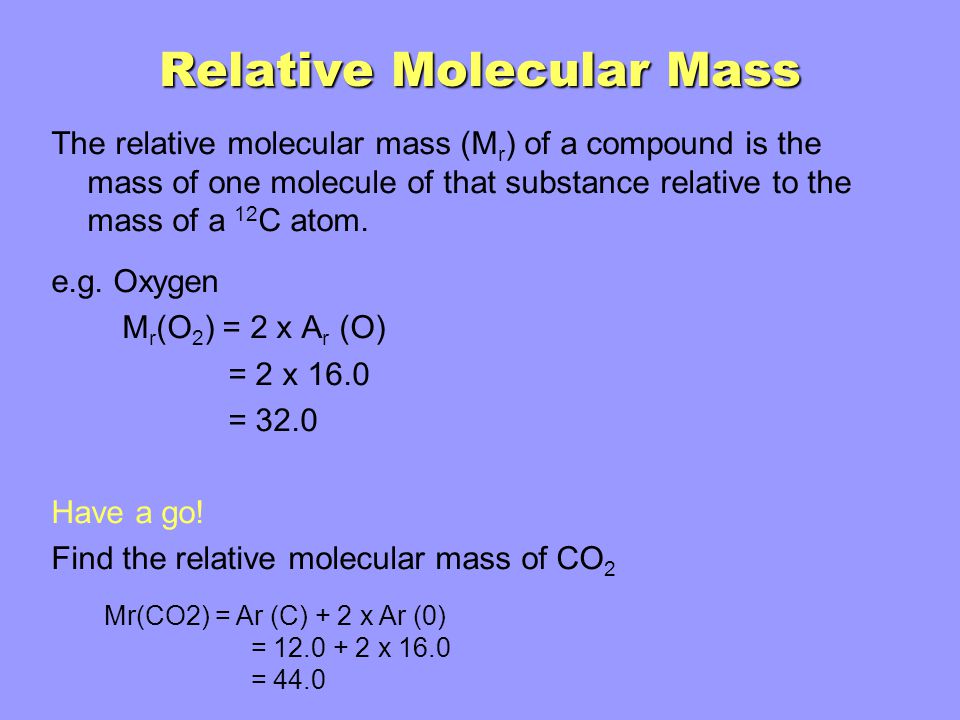
Relative Atomic Mass And The Mole Ppt Video Online Download

Week 3 Taste And Smell 3 1 Relative Molecular Mass Rmm Openlearn Open University Soa 1
This is defined as 0001 kilogram per mole or 1 gram per mole.
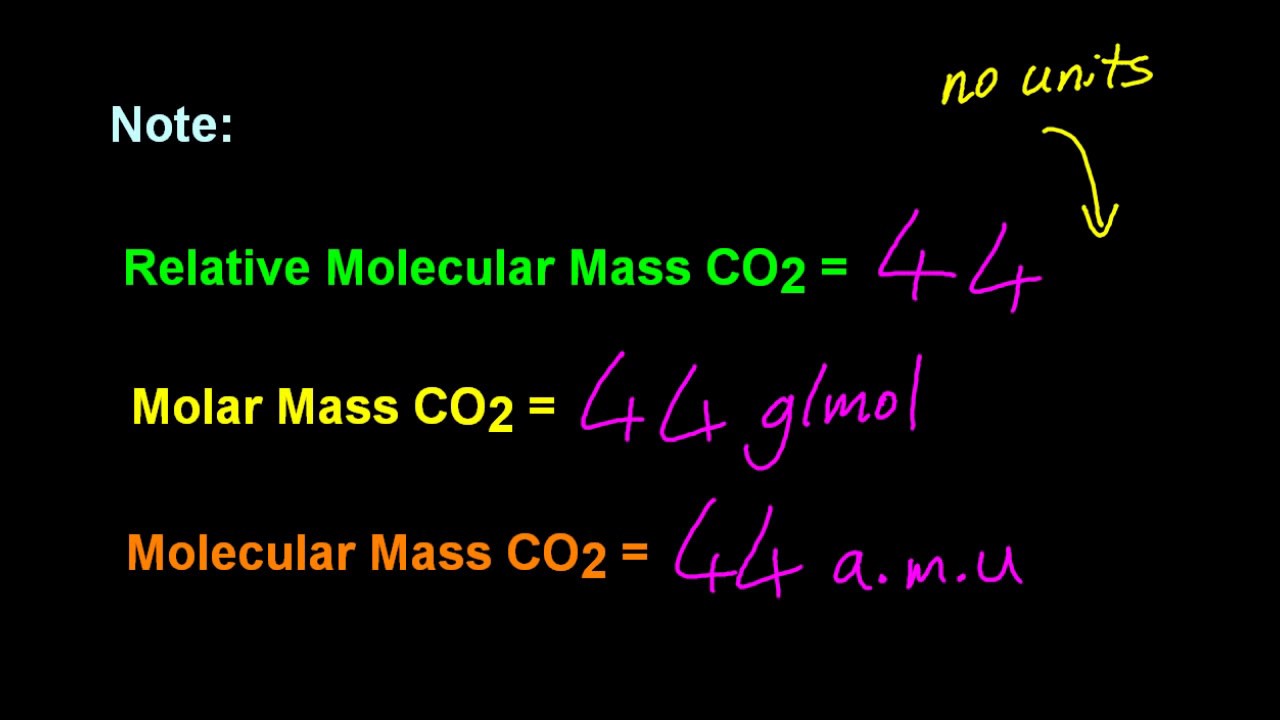
Relative molecular mass. The relative atomic mass scale is used to compare the masses of different atoms. Relative molecular mass M r and Relative formula mass F r Relative molecular mass of a substance is the average mass of a molecule of the substance when compared with one twelfth of the mass of one carbon-12 atom. It is often shortened to RMM.
Relative atomic mass synonyms relative atomic mass pronunciation relative atomic mass translation English dictionary definition of relative atomic mass. The smallest particle in an element or compound is a molecule that possesses the chemical properties of that element or compound. So the calculation of the molecular mass of SO 3 is required first.
Modern mass spectrometers easily distinguish resolve ions differing by only a single atomic mass unit amu and thus provide completely accurate values for the molecular mass of a compound. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. N the ratio of the average mass per atom of the naturally occurring form of.
Atomic and molecular masses are assigned relative to the mass of the carbon isotope 12 C whose atomic weight is defined as exactly 12. This simply means the calculation is performed using relative atomic weight values for the elements which are based on the natural isotopic ratio of elements found in Earths atmosphere and crust. These relative weights computed from the chemical equation are sometimes called equation weights.
Electrons have negligible mass and a negative charge. For example the relative molecular mass of water is 18. Calculations are based on.
Neutrons have a relative mass of 1 and are neutral. Molecular mass is a dimensionless quantity but it is given the unit Dalton or atomic mass unit as a means of indicating the mass is relative to 112th the mass of a. By definition the molar mass of SO 3 equals its molecular mass in grams.
The RMM is used in many sorts of calculations in chemistry and so you must be able to calculate it to answer all the other calculations you might meet. Not to mention the myriad of masses represented by all the metric prefixes to prepend to gram. The relative formula mass of a substance is the sum of the relative atomic masses of the elements present in a formula unit.
The symbol for relative formula mass is M r. Find the relative atomic mass of each element in the molecule. It is measured in daltons Da or u.
A r or atomic weight is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constantThe atomic mass constant symbol. For carbon dioxide CO 2 the relative atomic mass is 12011 amu for carbon and 15999 for oxygen. The molecular mass gives the mass of a molecule relative to that of the 12 C atom which is taken to have a mass of 12.
It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. What is relative formula mass and relative molecular mass. Relative Formula Mass Definition.
Use a copy of the Periodic Table of ElementsThe Periodic Table lists the atomic mass of each element below the chemical symbol. By using this chemists work out the chemical formula. Define relative atomic mass.
If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. What is the molar mass of sulphur trioxide SO 3 a molecular compound.
Since both quantities in the ratio are masses the resulting value is dimensionless. The following button will activate a random display of problems concerning the reactivity of common functional groups. Multiply the relative atomic mass by the molar mass constant.
This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole. Mass Percentages to Empirical Formula. All chemical ratios work just as well with masses as.
For example oxygen has a relative atomic mass of 159994 amu. Since the mass of an atom would be extremely small when measured in grams it would be more convenient to measure the masses of atoms relative to a standard atom. By knowing these we can follow the steps below to generate the empirical or molecular formula.
The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Convert mass percentages to the gram by considering 100 g of a sample. The molecular mass m is the mass of a given molecule.
M u is defined as being 1 12 of the mass of a carbon-12 atom. With the automotive industry moving towards automated driving sensing is increasingly important in enabling technology. The related quantity relative molecular mass as defined by IUPAC is the ratio of the mass of a molecule to the unified atomic mass unit also known as the dalton and is.
In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The standard atom chosen is carbon-12 isotope.
If the substance is made of simple molecules this mass may also be called the relative molecular mass. The highest-mass ion in a spectrum is normally considered to be the molecular ion and lower-mass ions are fragments from the molecular ion assuming the sample is a single pure compound. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight.
You can work chemistry mass problems in any mass you want and it will still work because the masses are relative to each other. In this paper virtual sensing is discussed for the case of. It is also known as the relative molecular mass is another because molecular weight calculated as the mass that is relative to the carbon -12 isotopes.
Relative atomic mass symbol. The Relative Molecular Mass of a compound is the sum of the masses of all the atoms present in the molecule. Full discussions of the topics covered by these problems are available in the Virtual Textbook of Organic Chemistry.
The mass of a molecule or an ion can be presented in daltons Da or kilodaltons kDa. This web application calculates the molecular mass average monoisotopic and nominal the elemental composition and the mass distribution spectrum of a molecule given by its chemical formula relative element weights or sequence. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
An atom of carbon-12 is taken to have a mass of 12 atomic mass unit amu. In practice the relative molecular mass of a compound M r is the sum of the relative atomic masses atomic weights of the atomic species as given in the chemical formula. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
Relative molecular mass of ammonia A r of nitrogen 3 x A r of hydrogen 1431 17. A related term you should know is relative formula mass relative formula weight. Through elemental analysis we can determine which elements are in a sample and their relative mass composition.
1 2 Define Relative Atomic Mass Ar And Relative Molecular Mass Mr Sl Ib Chemistry Youtube

Relative Molecular Mass Chemical Formulae And Equations Chemistry Youtube

1 2 1 Define The Terms Relative Atomic Mass A R And Relative Molecular Mass M R Youtube
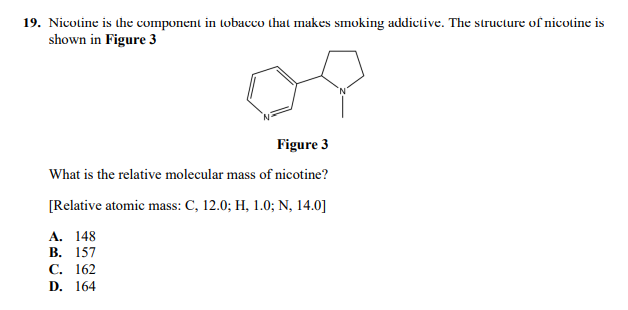
Answered 19 Nicotine Is The Component In Bartleby
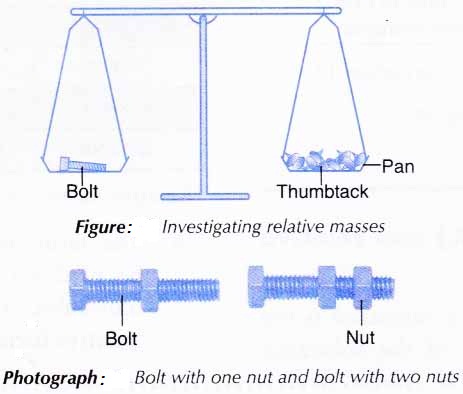
What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper

Relative Atomic Mass Molecular Mass O Level Chemistry Notes
Molecular Formula A Carbon Compound Chemistry Lovers Facebook
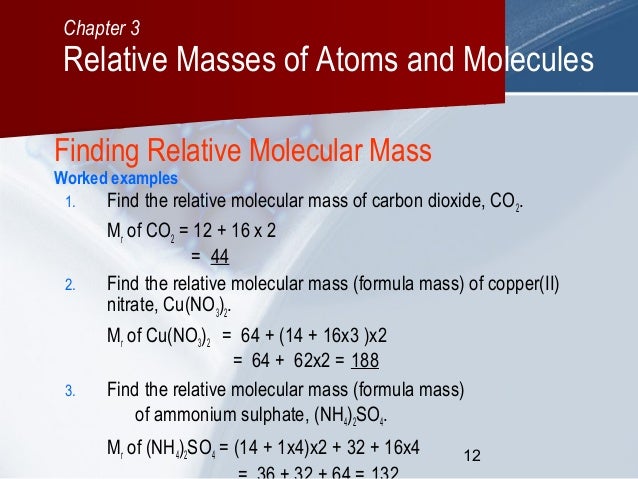
C03 Relative Masses Of Atoms And Molecules


